Amanda McFarland, a Senior Consultant at ValSource, Inc, and a member of the Kilmer Community Rapid Microbiology Methods group contributed to the following article presenting the first four steps of an initial evaluation roadmap for modern microbial methods.
This article presents the first four steps of an initial evaluation roadmap for modern microbial methods. These steps comprise the initial technology assessment, data and compliance risk, cost considerations and an overall instrument evaluation (See Figure 1).
Lessons learned and other industry commentary were used to outline a simplified set of steps to take when evaluating a modern technology. While the success of modern microbial method evaluation will largely depend upon the individuals tasked with the program, this roadmap provides information on high-level topics, which questions to ask and what internal stakeholders should be involved to minimize the challenges and set a foundation for success. This article is intended for a broad audience, including those involved in decision-making who may have limited experience with the evaluation and implementation of modern microbial methods.
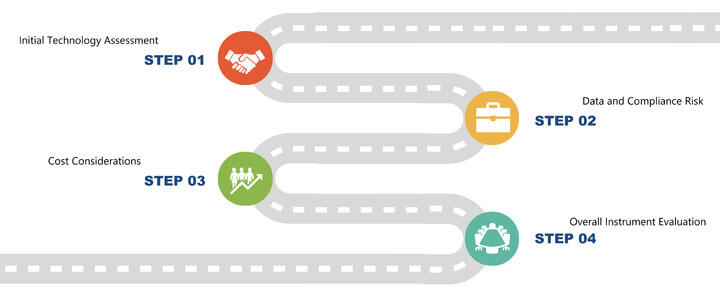 Figure 1 Evaluation Roadmap for Modern Microbial Methods
Figure 1 Evaluation Roadmap for Modern Microbial MethodsBackground
The use of modern microbial methods and advanced technologies have been encouraged for decades through industry guidance documents, like the 2004 PAT Guidance for Industry and Annex 1 (1,2). These technologies can provide a considerable benefit in the form of enhanced sensitivity, cost savings, time savings and continuous monitoring support, among others (3,4). Regulatory emerging-technology programs, like the U.S. FDA Center for Drug Evaluation and Research Emerging Technology Team, FDA Center for Biologics Evaluation and Research Advanced Technologies Team and European Medicines Agencies Innovation Task Force, have also been created to support use of new technologies. Such documents as USP General Chapter <1223>, Eur. Ph. Chapter 5.1.6 and PDA Technical Report No. 33 are also available to support the validation of alternative and rapid methods (5-7).
Despite the benefits that modern technologies can provide and the extensive regulatory guidance available, adoption of modern microbial methods has been a rare event in recent years. Challenges encountered during the assessment of new technologies can contribute to a lack of understanding of the initial evaluation and implementation process. One common oversight is not involving a comprehensive team of internal stakeholders early in the technology evaluation process (8). Within the context of this article, “modern microbial methods’’ are synonymous with alternative microbial methods and rapid microbial methods.
Step 1: Initial Technology Assessment
When determining which modern microbial method to investigate, several questions should be considered:
- Would use of a new technology align with company goals and needs?
- What application would benefit from use of a modern technology?
- What are the specific goals of the project team?
- Has the company already experienced a candidate technology?
If shelf life is of concern, a rapid release test for sterility or mycoplasma may be the appropriate type of technology to investigate further. Other applications may include in-process testing of incoming raw materials or water; continuous environmental monitoring for air, water and/or surfaces; data integrity; or rapid return from shutdown or maintenance. Although not required, it is helpful if the strategy for development and implementation can begin when the product is in clinical trials, so the New Drug Application or Biologics License Application includes this alternative method for testing. Some companies are evaluating modern microbial methods for new facilities to increase the confidence in their overall quality, to gather data from the time of facility-commissioning and to use data to drive decisions and optimize processes. Furthermore, modern microbial methods can be excellent tools for non-GMP investigations and validations, such as for routine testing or monitoring, prior to implementation in the final application. Considering the applicability of the technology to the target application and evaluating other use cases of the technology that can provide value as the target implementation work is conducted may also be a benefit.
Technical Considerations
Considerations for maturity of the technology must be made. For example, in circumstances where only the basic principles of the technology have been observed and reported, additional studies may be required to confirm the concept application. This may include demonstrating critical functions, verification studies and a risk assessment to generate supporting data. In circumstances where a technology has been adopted for the intended application in a different laboratory setting, the verification phase may be streamlined based on a risk assessment and published literature.
The intended use of the technology can bring about more specific technical considerations and questions:
- What is the time to result, or is it continuous?
- Is the time-savings in hours or days?
- What is the risk of false-positive and/or false-negative results, as compared to existing methods?
A risk assessment should be performed to consider the vulnerabilities associated with traditional methods compared to the adoption of a modern microbial method. This assessment will form the basis for method-development and validation-work packages and will better inform the potential value of the technology.
Critical attributes, like sensitivity of the modern microbial method, may be better than the traditional or compendial method. It is critical to understand the unit in which the modern technology reports, if it is different from the colony-forming unit, and how this sensitivity relates to the method currently in use. Some questions or points to consider include the following:
- Does the technology have published papers or vendor data demonstrating general detection performance and sensitivity to a range of organisms that are appropriate to the application?
- What is the required limit of detection for the application? The end user may need to demonstrate that the modern microbial method has a detection limit that is equivalent to or better than the current one.
- If the application involves product, interference and background must be understood with some technologies.
- Does the supplier have extensive data and the ability to support the primary validation of the technology and method suitability testing? (5-7)
The abilities of the modern microbial method and the way the technology will supplement or replace methods currently in place are also important to understand. Depending on the application, microorganism identification may be critical in the event of an out-of-specification result. If the modern microbial method is destructive to some microbes, the question arises: How will this be addressed during an investigation? One option is to continue to use growth-based methods side by side to trend, understand and then respond accordingly. It should be determined if side-by-side testing is appropriate to the modern microbial method and application selected (e.g., end-product versus in-process testing) or is suggested by the local regulatory authority. A goal for the implementation of a new technology may be to simply enable a reduction in the use of a traditional method or enable data-driven decision-making about when to perform additional testing.
Step 2: Data and Compliance Risk
An understanding of data reported by the system and how data will be retrieved and analyzed is also important during the evaluation of any technology. All new instruments being evaluated for GMP use should meet the following minimum acceptable parameters:
- Support 21 CFR Part 11 compliance
- Create a data record that is uneditable by the user
- Contain an uneditable activity log that records user actions
- Contain an alarm matrix able to capture any invalid occurrence
- Be able to interface with the company’s active directory
- Be able to interface with electronic data management systems and be able to export data and/or reports in a readable format
- Include options for data export that fit within company control policies
- Prevent a user at any permission level to edit or delete a data record without generating a quality-event record
- Contain enough user permission levels and allow custom configuration of user permissions consisting of, at minimum, an administrator, and an operator
In addition to the outlined parameters discussed above, it is important to develop a working relationship with the instrument vendor and to involve IT and other relevant internal stakeholders familiar with these data and compliance requirements. Vendors that can provide resources to support qualification and integration within any internal existing systems should be preferred.
Step 3: Cost Considerations
Cost is considered at each phase of implementation, including during routine use. For initial discussions, all information related to total cost of ownership should be gathered and procurement must be involved in early discussions to enable negotiations for competitive pricing. If multiple sites are impacted, the global procurement function needs to be included to ensure that the best pricing is attained, and an aligned strategy (for both pricing and implementation) is shared across the network. Additional stakeholders who need to be informed or consulted during this process include the receiving labs, project manager, project sponsor and applicable leadership.
What exactly must be considered for the total cost of ownership? This can be viewed holistically as capital expenditures (CAPEX) and operational expenditures (OPEX) or, more simply, as the initial and long-term costs. These initial and long-term costs can be narrowed down to the aspects outlined in Table 1.
Table 1 Initial and Long-term Costs of Modern Microbial Methods
| Initial (One-Time) | Long-Term (Recurring) |
|---|---|
| Cost of instrument Cost of supporting software or equipment |
Cost of maintenance plan (based on defined frequency/service level) |
| Cost of any engineering or structural lab updates (e.g., minor/major construction, piping, HVAC) | Cost for system updates/upgrades and replacement |
| Cost of validation package (e.g., installation, operational and performance qualification) | Cost of consumables and continued training |
Supplier agreements should be expected to include clear benefits (e.g., decrease in cost) as the number of instruments and consumables purchased increases over time.
The project timeline should include elements that ensure the deployment of sufficient resources, either internal or external, and the accounting for any associated expenses. The vendor should be engaged to determine the timeline for when the equipment will be received, installed, and qualified and when the applicable training will be performed. Vendor support for regulatory submissions should be included, if applicable, and aligned with the internal regulatory group for impact to current licenses and cost associated for any updates.
Step 4: Overall Instrument Evaluation
After the first three steps are complete, a final overall instrument evaluation, or summary report, can be compiled based on the knowledge and feedback gathered. The goal is to determine if the instrument supports current business needs and/or future goals and if the modern microbial method and the company for integration of this new technology are ready to employ it. This includes a summary of the technology, communication on how well the instrument meets the requirements of internal stakeholder groups, an initial evaluation of the supplier and the willingness of the team to support next steps with the technology.
Summarizing the abilities of the new technology and potential uses within the company are important for ensuring realistic expectations and communicating the technology’s potential benefits. The time required to reach this goal may be considerable, but the technology can provide numerous other benefits and uses along the way. For example, biofluorescent particle-counting systems can be used as continuous-monitoring process-control tools that support root-cause assessment and faster investigation resolution (9). As part of the final instrument evaluation, it is beneficial to summarize the technology’s capabilities, its intended uses, the extent of required validation testing, the length of time that the modern microbial method has been used in the industry, including relevant example use cases, and the industry support for the technology (e.g., end-user groups or other colleagues) (3,4,8,9).
As a next step, the final assessments or conclusions should be reviewed and confirmed with subject matter experts from each relevant internal stakeholder group. These groups may include IT/data support, statisticians, inspection readiness/regulatory team, automation team, quality assurance, quality control, procurement, maintenance, utilities and global functions. It is important to bring on board stakeholders who will be working with, be affected by, or will benefit from the technology as early as possible to minimize the potential for issues later. Questions to consider include the following:
- Does the instrument meet compliance and data-integrity standards?
- How much training is required to use the equipment and what level of expertise is needed?
- Is there a projected return-on-investment for the instrument?
- Does the time to implement fit into company goals?
- What changes are required to implement the technology?
- What level of method-validation testing and support is required?
A summary report can address concerns expressed by these groups during steps one through three to further support the recognition of internal stakeholder requirements and concerns.
An evaluation of the supplier is an important element of an overall equipment evaluation. Companies should consider:
- What is the vendor’s willingness and ability to offer support during the evaluation and implementation of the technology?
- Does the vendor offer onsite service, and what is the typical response time?
- Does the vender have a robust supply chain, change control and quality system in place?
- What Is the potential for proprietary or single-source consumables to be out of stock, affecting the ability to use the technology?
Answering all supplier-related questions may not be immediately possible, but it is important to start discussing such questions with the vendor early in the process. Other users of the technology may also provide good resources in this aspect of the evaluation.
Finally, it is helpful to compile information on an initial installation location and on a project team who is willing to support an onsite evaluation of the instrument, considering the following:
- What is the need at the site that the instrument could help fulfill?
- Why is this an optimal location for initial installation and are there any potential concerns?
- Does the location have the environment to support the instrument? Specifically, will the instrument fit? Is there electrical and network support available? Is a drain required?
Finally, once a successful onsite evaluation has been conducted, a champion should be identified within the project team who is willing to drive the pilot project.
Conclusion
Benefits from adopting modern microbial methods can be seen across the microbiological control strategy. Adopting new technology may facilitate enhancements to data integrity, process robustness, process understanding and the ability to respond to drifts in real time, rather than waiting several days to discover that a problem exists via traditional techniques. Currently, traditional microbiological methods offer a fit-for-purpose solution to microbial quality assurance. As products evolve and patient reach expands, technologies that can enhance awareness and decrease the time to result become available. This improves the current state of limited data that are available during the microbial review of manufacturing environments, processes and products.
The hurdle to adopt modern microbial methods may seem large; however, preparing a clear framework to adopt such methods, like the one outlined here, can reduce any obstacles. Users must understand the limitations of current methods and assess those risks prior to adoption of a new method. Steps to mitigate those risks can then be taken during the development of an implementation plan for the new technique. Following the guidance outlined by PDA and the major pharmacopoeias, implementation and use of 21st century technologies in the microbiology laboratory is possible.
References
- U.S. Food and Drug Administration. Guidance for Industry PAT – A Framework for Innovative Pharmaceutical Development, Manufacturing and Quality Assurance, Sept. 2004.
- European Commission. Annex 1: Manufacture of Sterile Products (Second Targeted Stakeholders’ Consultation on Draft Revision 12), EudraLex – Volume 4, Feb. 2020. https://ec.europa.eu/health/medicinal_products/consultations/2020_sterile_medicinal_products_en (accessed Aug. 15, 2021).
- Moldenhauer, J. The Rush to Rapid Microbiological Methods – Or Not. Eur. Pharm. Rev. 2017, Issue 2. https://www.europeanpharmaceuticalreview.com/article/77421/the-rush-to-rapid-microbiological-methods-or-not/ (accessed Oct. 28, 2021).
- Miller, M. The Encyclopedia of Rapid Microbiological Methods: The New Fourth Volume Discusses Technologies, Regulatory Acceptance and Validation Case Studies. Eur. Pharm. Rev. 2013, Issue 3. https://www.europeanpharmaceuticalreview.com/article/19408/the-encyclopedia-of-rapid-microbiological-methods/ (accessed Oct. 28, 2021).
- U.S. Pharmacopeial Convention. General Chapter <1223> Validation of Alternative Microbiological Methods. In USP 42–NF 37; USP: Rockville, Md. 2016.
- Parenteral Drug Association. Technical Report No. 33 (Revised 2013): Evaluation, Validation and Implementation of Alternative and Rapid Microbiological Methods; PDA: Bethesda, Md., 2013.
- Council of Europe. Alternative Methods for Control of Microbiological Quality Chapter, 5.1.6. In European Pharmacopoeia 10.0, Council of Europe: Strasbourg, 2019; p 628.
- Paul, M.A.; et. al. Alternative and Rapid Micro Methods (ARMM): Framework for the Evaluation, Validation and Implementation of Alternative and Rapid Microbiological Testing Methods. BioPhorum Operations Group, Jul. 2020.
- Prasad, A., et. al. Biofluorescent Particle Counter-Based Real-Time Feedback and Control of Processing Conditions. Eur. Pharm. Rev. 2019, Issue 4. https://www.europeanpharmaceuticalreview.com/article/98107/biofluorescent-particle-counter-based-real-time-feedback-and-control-of-processing-conditions/ (accessed Oct. 28, 2021).